Ozone is made up from three oxygen atoms, whereas the oxygen that we breathe has only two. It is a minor gas in our atmosphere and mostly occurs in the “ozone layer” at heights between 10 and 35 kilometres. Bringing all the ozone in the “layer” down to ground level would give a thickness of around 3mm of pure ozone, which reduces to around 1mm at the height of the ozone hole. At the surface of the earth it is poisonous in large quantities, but in the stratosphere it prevents harmful ultra-violet radiation from reaching us.
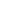
The Ozone Hole
The discovery by the British Antarctic Survey of the Antarctic ozone hole provided an early warning of the dangerous thinning of the ozone layer worldwide, and spurred international efforts to curb the production of CFCs. The provisions of the Montreal Protocol of 1987 on Substances that Deplete the Ozone Layer have been revised and strengthened and are being followed by virtually all UN Member states. There is a reasonable prospect that the Antarctic ozone hole will permanently repair itself, but not until around 2070.
British scientists began their measurements of Antarctic ozone at Halley in 1956. The aim was to understand the important role that ozone plays through absorbing solar energy, in determining the temperature profile of the stratosphere and its wind circulation.
The amount of ozone overhead Halley station follows a regular seasonal pattern. In the spring, ozone amounts begin to fall and reach a minimum in early October — this is the peak of the Antarctic ozone hole. In late spring ozone amounts rise to a maximum and then slowly decline.
The spring-time ozone hole is the result of emissions, mainly in the northern hemisphere, of chlorofluorocarbons (CFCs) and halons. These gases were in widespread use in refrigeration, industrial solvents and fire control, but are now regulated by the Montreal Protocol. The gases are broken down into their constituents over the tropics, and circulate towards both poles. Only over the Antarctic during winter is it cold enough for clouds to form in the ozone layer, and here chemical reactions on the cloud surfaces convert chlorine into an active form. When the sunlight comes back in the spring, this activated chlorine destroys ozone at about 1% per day leading to the ozone hole. As the atmosphere warms, the clouds disappear and the ozone hole fills in.